Arterial thrombosis is a condition targeted for treatment by many therapeutic drugs. However, currently, every drug used to treat this condition lead to an increased risk of bleeding - specifically of bleeding disorders. Why exactly is this? In simple terms, antiplatelet drugs have potent effects on the final common pathway of platelet aggregation. Platelet aggregation is a process whereby platelets adhere (stick) to each other at sites of vascular (related to/affecting/consisting of a vessel or vessels) injury, and is critical for the formation of haemostatic plugs, and blood clots which prevent bleeding when you have a wound. Arterial thrombosis is a condition where a thrombus (blood clot) is found in an artery. Typically, its primary cause is vessel disease, which in turn leads to the rupture of an atherosclerotic plaque. If this occurs in an artery that supplies the heart, it can cause a heart attack. If it occurs in an artery that supplies the brain, then it can result in stroke. There are many risk factors associated with the predisposal to a greater chance of developing arterial thrombosis - including both environmental and lifestyle factors - such as obesity, smoking and high blood cholesterol, as well as inherited factors. The familial tendency for developing arterial thrombosis is strong, with multiple genes showing as responsible for its development. Antiplatelet drugs are used to decrease platelet aggregation, and thus inhibit the formation of thrombi.
The aims of modern platelet research are balanced between the prevention of heart attack and stroke through the inhibition of platelet activation, while ensuring that platelets are not inhibited entirely in order to prevent bleeding complications for the patient.
Current Treatments
Standard treatments for arterial thrombosis currently use aspirin and clopidogrel. Prasugrel is also being used instead of clopidogrel to block the P2Y12 G-protein coupled receptor (GPCR) which is proving to be more effective at reducing the risk of stroke and heart attack. However, it does carry an increased bleeding risk compared with clopidogrel. Contrarily, aspirin targets a component of the thromboxane pathway called cyclooxygenase. Other drugs targeted to inhibit the αIIbβ3 integrin (such as eptifibatide and abciximab) have also been developed - these are seen to be used during stenting procedures to open up blood vessels where restriction through means of an atherosclerotic plaque or otherwise has occurred. Though, as a result of high bleeding risk associated with their use, they are not used for long term treatments. Anticoagulants can suppress platelet activation due to their ability to directly inhibit thrombin, and hence some of these can also be used in the treatment of arterial thrombosis.
So, what are these components? What are we actually targeting?
- P2Y12 G-protein coupled receptor (GPCR): a GPCR is a cell surface receptor which acts to detect messages through signalling molecules such as peptides, light energy, lipids and sugars to inform the cell about its environment. They consist of a single globular polypeptide, with seven segments that span the entire width of the membrane, with intervening portions looping inside and outside of the cell (shown in Figure 1). GPCRs interact with G proteins in the plasma membrane. When a signalling molecule binds the GPCR, a conformational change is induced, thus triggering an interaction between the GPCR and a nearby G protein. P2Y12 is a chemoreceptor (chemical receptor) for adenosine diphosphate (ADP).
- Thromboxane A2 (TXA2; GPCR): also a GPCR. TXA2 is a potent stimulator of platelet aggregation, and its activity is mediated through a G protein that activates a phosphatidylinositol-calcium second messenger system. Aspirin targets this particular receptor.
- αIIbβ3 integrin: integrins are transmembrane glycoprotein (proteins modified through the addition of lipid) which are able to transmit information both ways across a plasma membrane (bidirectional transfer). αIIbβ3 is expressed at high levels in platelets are progenitors of platelets, playing a key role in platelet function, haemostasis and arterial thrombosis. It also participates in cancer progression through tumour cell proliferation amongst other systems. In resting platelets it has an inactive conformation, but upon stimulation through agonists, the transduction of signals leads to a switch to a high affinity state particularly for fibrinogen. Overall this causes integrin clustering and hence drives aggregation, thrombus consolidation and more. Hence drugs to target this integrin are produced.
- von Willebrand Factor (vWF): vWF is an adhesive and multimeric glycoprotein necessary for normal haemostasis. It has central role in many processes including the mediation of platelet adhesion to vascular sub-endothelium, and therefore also platelet aggregation. Hence, drugs to target vWF and its respective integrin (shown in Figure 1) are in development through clinical trials.
- Collagen: appears in a platelet aggregation pathway, calcium dependent. An increase in collagen leads to an increase in the concentration of calcium present in the platelets, and hence an increase in platelet aggregation. The increase in platelet aggregation tends to be seen as a result of calcium influx from the sodium-calcium exchanger functioning in a reverse mode from the extracellular milieu. Hence, drugs are in development and trials for targeting this too.
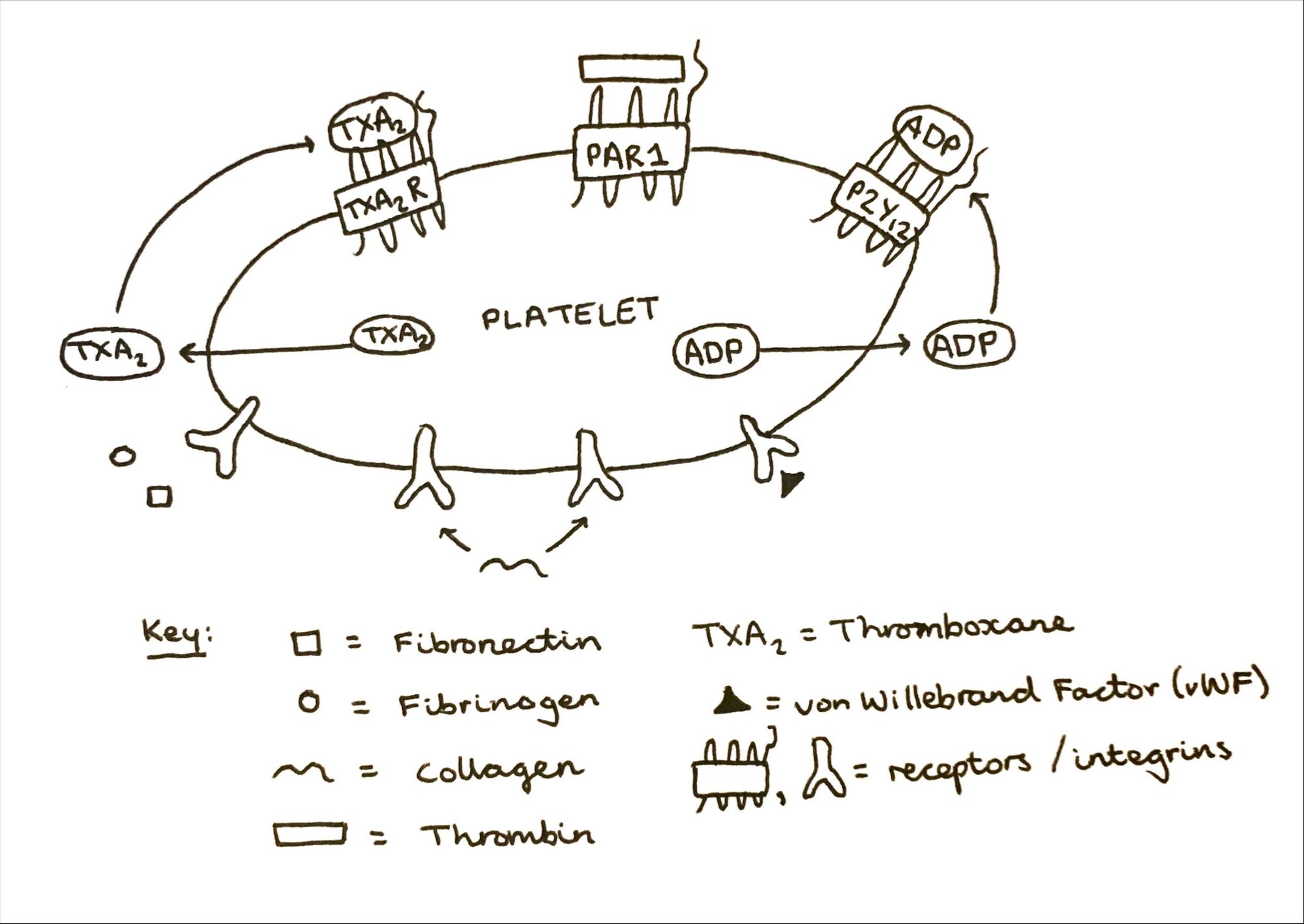
As shown above in Figure 1, antiplatelet therapy often refers to a dual therapy combining aspirin and a P2Y12 GPCR inhibitor (clopidogrel/prasugrel) which in turn targets the activation of platelets via thromboxane and ADP. These dual therapies significantly reduce the risk of cardiovascular events compared to monotherapy or lack of treatment, but the elevated risk of major bleeding is something to also be considered. The bleeding risk is also dependent on other factors including the duration of therapy and dosage(s) prescribed.
Modern Approaches
As a result of the high bleeding risks associated with the current primary treatments, the search for novel antiplatelet drugs that have a high efficacy with respect to the treatment of arterial thrombosis, whilst having a lower (or better still, if possible, nil) bleeding risk is a key aim of current research involving platelets.
vWF, and its integrin GPIbα-GPIX-GPV (and the overall pathway associated with adhesion). vWF has been shown to be important in platelet adhesion and aggregation whereby the GPIb-vWF complex contributes to the tethering and rolling of the platelet to the vessel. This is outlined in Figure 2. People who are deficient in vWF have von Willebrand disease bleeding disorder. Antiplatelet drugs made to target this factor and integrin will enable the risk of thrombosis to be decreased as a result of preventing platelet tethering. However, theoretically this could increase the risk of bleeding shown by vWF-deficient humans.

However, it has been found in early stage (phase I) clinical trials (Cataland et al., 2012) that one particular drug, ARC1779, shows improvements in clinical symptoms - particularly the reduction of cardiovascular events. However, as of yet no issues have arisen with respect to patient safety, including bleeding. Hence, there is the possibility that drugs such as ARC1779, or those that target in similar ways could lead to a reduction in bleeding risks for patients. Despite this, these are early stage trials and further clinical trials on larger scales with many more patients will be required in order to see whether they could be of significant benefit when used as an anti-platelet therapy since phase I trials only evaluate the efficacy of a drug within a small patient cohort, so the effects on a large population cannot yet be evaluated - including the possibility of significant adverse events that may occur in 1 in 50 patients for example. Other drugs that target vWF, or collagen will also have to go through the safe processes. At the moment, treatments focus on these factors found within the membranes of platelets and hence there are a limited number of factors to observe and target. Regardless, it may be possible to find something which significantly reduces bleeding risk whilst still being effective, or there may be other ways which have not yet been considered such as further back in signalling networks and targeting the signalling molecules themselves that are attracted to the receptors.
Nonetheless, this progress is promising; showing that it may be possible to produce a drug with a significantly reduced risk of bleeding, even if the bleeding still occurs somewhat, especially when compared with the current treatments available. Furthermore other actions can be considered, such as if a drug is highly potent, using a lower concentration or dosage which may also decrease the risk of bleeding for the patient. Although, this cannot afford to compromise the efficacy of the drug since in many cases a lower concentration may not actually prevent thrombosis, or it could lead to a patient developing resistance to the drug before it has been able to treat the condition for a significant period of time in more circumstances due to exposure at lower dosages. However, if the drug is highly potent, it could therefore still be possible.
Conclusion
In conclusion, there are signs (as evidenced above) that antiplatelet therapy whereby less bleeding is experienced, at least in a majority of patients, may be possible to be developed. However, eliminating the risk of bleeding in its entirety seems rather unlikely at the moment. A number of factors that are the subject of new developments and possible future treatments, including vWF, are essential in the adhesion and aggregation of platelets to prevent bleeding disorders. As a result, the targeting of these in any way could lead to disruption of these normal processes such as seen through thromboxane inhibitors leading to increased bleeding risks. Drugs such as ARC1779 show potential with further study, especially if the drugs can be administered at low dosages due to high potency. Another possibility would be the drug being able to treat arterial thrombosis without triggering a vWF deficiency and hence reducing bleeding risk.
I hope that through this article I've been able to outline some of the key ideas related to treating arterial thrombosis using antiplatelet drugs - including a focus on trying to reduce the risk of bleeding disorders if possible. If you are interested in this area, feel free to ask questions in the "Any Questions?" page and I will reply as soon as possible! Upcoming article topics include caspase 8, and antimicrobial resistance, and will be far more frequent now that my exams are over. Stay tuned for more!
References
Algahtani, F. H. and Stuckey, R. (2019) ‘High factor VIII levels and arterial thrombosis: illustrative case and literature review’, Therapeutic Advances in Hematology, 10, p. 204062071988668. doi: 10.1177/2040620719886685.
Cataland, S. R. et al. (2012) ‘Initial experience from a double-blind, placebo-controlled, clinical outcome study of ARC1779 in patients with thrombotic thrombocytopenic purpura’, American Journal of Hematology, 87(4), pp. 430–432. doi: 10.1002/ajh.23106.
Eikelboom, J. W. et al.(2005) ‘Enhanced antiplatelet effect of clopidogrel in patients whose platelets are least inhibited by aspirin: a randomized crossover trial’, Journal of Thrombosis and Haemostasis, 3(12), pp. 2649–2655. doi: 10.1111/j.1538-7836.2005.01640.x.
Fan, H. et al. (2019) ‘Structural basis for ligand recognition of the human thromboxane A2 receptor’, Nature Chemical Biology, 15(1), pp. 27–33. doi: 10.1038/s41589-018-0170-9.
Ferraris, V., Ferraris, S. and Saha, S. (2011) ‘Antiplatelet Drugs: Mechanisms and Risks of Bleeding Following Cardiac Operations’, International Journal of Angiology, 20(01), pp. 001–018. doi: 10.1055/s-0031-1272544.
Fontana, P. et al. (2014) ‘Antiplatelet Therapy: Targeting the TxA2 Pathway’, Journal of Cardiovascular Translational Research, 7(1), pp. 29–38. doi: 10.1007/s12265-013-9529-1.
Gimbel, M. et al. (2020) ‘Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): the randomised, open-label, non-inferiority trial’, The Lancet, 395(10233), pp. 1374–1381. doi: 10.1016/S0140-6736(20)30325-1.
Gurbel, P. A., Kuliopulos, A. and Tantry, U. S. (2015) ‘G-Protein–Coupled Receptors Signaling Pathways in New Antiplatelet Drug Development’, Arteriosclerosis, Thrombosis, and Vascular Biology, 35(3), pp. 500–512. doi: 10.1161/ATVBAHA.114.303412.
Hao, Q. et al. (2018) ‘Clopidogrel plus aspirin versus aspirin alone for acute minor ischaemic stroke or high risk transient ischaemic attack: systematic review and meta-analysis’, BMJ, p. k5108. doi: 10.1136/bmj.k5108.
Huang, J. et al. (2019) ‘Platelet integrin αIIbβ3: signal transduction, regulation, and its therapeutic targeting’, Journal of Hematology & Oncology, 12(1), p. 26. doi: 10.1186/s13045-019-0709-6.
Leebeek, F. W. G. (2019) ‘A prothrombotic von Willebrand factor variant’, Blood, 133(4), pp. 288–289. doi: 10.1182/blood-2018-11-883488.
Peyvandi, F., Garagiola, I. and Baronciani, L. (2011) ‘Role of von Willebrand factor in the haemostasis’, Blood Transfusion, pp. s3–s8. doi: 10.2450/2011.002S.
Rana, A. et al. (2019) ‘Shear-Dependent Platelet Aggregation: Mechanisms and Therapeutic Opportunities’, Frontiers in Cardiovascular Medicine, 6, p. 141. doi: 10.3389/fcvm.2019.00141.
Roberts, D. E., McNicol, A. and Bose, R. (2004) ‘Mechanism of Collagen Activation in Human Platelets’, Journal of Biological Chemistry, 279(19), pp. 19421–19430. doi: 10.1074/jbc.M308864200.
Rosenbaum, D. M., Rasmussen, S. G. F. and Kobilka, B. K. (2009) ‘The structure and function of G-protein-coupled receptors’, Nature, 459(7245), pp. 356–363. doi: 10.1038/nature08144.

